Гәрәбә әчелеге
| Гәрәбә әчелеге | |
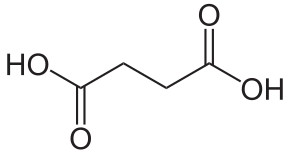 | |
| Масса | 118,027 м.а.б.[1] |
|---|---|
| Бәйле нигез | succinate(1-)[d] |
| Химик фурмула | C₄H₆O₄[1] |
| SMILES фурмуласы | C(CC(=O)O)C(=O)O[1] |
| Эрү температурасы | 187 °C[2] һәм 188 °C[3] |
| Нинди таксонда бар | Ошибка Lua в Модуль:Sources на строке 178: attempt to concatenate local 'letter' (a nil value). |
Гәрәбә әчелеге (НООС-СН2-СН2-СООН) - ак кристаллар; тозлары һәм эфирлары сукцинатлар дип атала. Аз күләмдә гәрәбә, көрән күмер һ.б.н. эчендә бар. Синтетик сулмалаларны алу өчен, органик синтезда кулланыла.
Искәрмәләр[үзгәртү | вики-текстны үзгәртү]
- ↑ 1,0 1,1 1,2 succinic acid
- ↑ Bradley J., Williams A., Andrew S.I.D. Lang Jean-Claude Bradley Open Melting Point Dataset // Figshare — 2014. — doi:10.6084/M9.FIGSHARE.1031637.V2
- ↑ David R. Lide, Jr. Basic laboratory and industrial chemicals: A CRC quick reference handbook — CRC Press, 1993. — ISBN 978-0-8493-4498-5
- ↑ Kurkin V. A., Zapesochnaya G. G., Klyaznika V. G. Flavonoids of the rhizomes ofRhodiola rosea. I. Tricin glucosides // Chemistry of Natural Compounds — Springer Science+Business Media, 1982. — ISSN 0009-3130; 1573-8388 — doi:10.1007/BF00575035
- ↑ DP Z. Dong quai // Am. J. Chin. Med. — World Scientific, 1987. — ISSN 0192-415X; 1793-6853 — doi:10.1142/S0192415X87000151 — PMID:3425569
- ↑ Upton R. Dong Quai — 2013. — doi:10.1201/B14669-29
- ↑ Copper = Leslie M. Klevay — 2013. — doi:10.1201/B13959-20
- ↑ M. Kloss, K.-H. Iwannek, I. Fendrik et al. Organic acids in the root exudates of diplachne fusca (linn.) beauv. // Environmental and Experimental Botany — Elsevier BV, 2003. — ISSN 0098-8472; 1873-7307 — doi:10.1016/0098-8472(84)90020-0
- ↑ C. Barbas, J. A. Lucas García, F. J. Gutiérrez Mañero Separation and identification of organic acids in root exudates ofLupinus luteus by capillary zone electrophoresis // Phytochem. Anal. — Wiley, 2005. — ISSN 0958-0344; 1099-1565 — <55::AID-PCA437>3.0.CO;2-I doi:10.1002/(SICI)1099-1565(199903/04)10:2<55::AID-PCA437>3.0.CO;2-I
- ↑ Y.S. Lewis, S. Neelakantan (−)-Hydroxycitric acid—the principal acid in the fruits of Garcinia cambogia desr. // Phytochemistry — Elsevier BV, 1965. — ISSN 0031-9422; 1873-3700 — doi:10.1016/S0031-9422(00)86224-X
- ↑ Khan F., Peter X. K., Mackenzie R. M. et al. Venusol from Gunnera perpensa: structural and activity studies // Phytochemistry — Elsevier BV, 2004. — ISSN 0031-9422; 1873-3700 — doi:10.1016/J.PHYTOCHEM.2004.02.024 — PMID:15110692
- ↑ Callahan D. L., Roessner U., Richard A. O'Hair et al. LC-MS and GC-MS metabolite profiling of nickel(II) complexes in the latex of the nickel-hyperaccumulating tree Sebertia acuminata and identification of methylated aldaric acid as a new nickel(II) ligand // Phytochemistry — Elsevier BV, 2008. — ISSN 0031-9422; 1873-3700 — doi:10.1016/J.PHYTOCHEM.2007.07.001 — PMID:17765935
- ↑ Hicks L. M., Gargouri M., Juergens M. T. The response of Chlamydomonas reinhardtii to nitrogen deprivation: a systems biology analysis // The Plant Journal — Wiley-Blackwell, 2015. — ISSN 0960-7412; 1365-313X — doi:10.1111/TPJ.12747 — PMID:25515814
- ↑ Chemical constituents from Artemisia annua // 中国中药杂志 — 2014. — ISSN 1001-5302 — doi:10.4268/CJCMM20142423
- ↑ SUGAR BEET (Beta vulgaris) — 2008. — doi:10.1007/978-1-4020-4585-1_2617
- ↑ Chemical constituents from Bidens bipinnata // 中国中药杂志 — 2014. — ISSN 1001-5302 — doi:10.4268/CJCMM20141017
- ↑ 17,0 17,1 Sun H. Flavonoids from Bidens pilosa var. radiata // Phytochemistry — Elsevier BV, 1997. — 4 p. — ISSN 0031-9422; 1873-3700 — doi:10.1016/S0031-9422(97)80026-X
- ↑ W Jia Flavonoids from Bidens pilosa var. radiata // Phytochemistry — Elsevier BV, 2002. — ISSN 0031-9422; 1873-3700 — doi:10.1016/S0031-9422(97)00420-2
- ↑ M.-Y Ding, P.-R Chen, G.-A Luo Simultaneous determination of organic acids and inorganic anions in tea by ion chromatography // J. Chromatogr. A — Elsevier BV, 2002. — ISSN 1873-3778; 0021-9673 — doi:10.1016/S0021-9673(96)00910-7
- ↑ Pei Y., Yang Z. D., Sheng J. Chemical Constituents of Anabasis salsa // Chemistry of Natural Compounds — Springer Science+Business Media, 2014. — ISSN 0009-3130; 1573-8388 — doi:10.1007/S10600-014-1132-4
- ↑ MIYASE T. Studies on the constituents of Cistanchis Herba. II. Isolation and structures of new iridoids, cistanin and cistachlorin. // Chemical & Pharmaceutical Bulletin — Pharmaceutical Society of Japan, 1984. — ISSN 0009-2363; 1347-5223 — doi:10.1248/CPB.32.1729
- ↑ Chu W., Gao P., Li L. Chemical constituents from the leaves of Crataegus pinnatifida Bge // Biochem. Syst. Ecol. — Elsevier BV, 2019. — ISSN 0305-1978; 1873-2925 — doi:10.1016/J.BSE.2019.103923
- ↑ Chemical constituents from tubers of Cremastra appendiculata // 中国中药杂志 — 2014. — ISSN 1001-5302 — doi:10.4268/CJCMM20140217
- ↑ T Komori Glycosides from Dioscorea bulbifera // Toxicon — Elsevier BV, 1997. — 6 p. — ISSN 0041-0101; 1879-3150 — doi:10.1016/S0041-0101(97)00032-9 — PMID:9428100
- ↑ Shuai S. Flavonoids of Erigeron canadensis // 中国中药杂志 — 2012. — ISSN 1001-5302 — doi:10.4268/CJCMM20121914
- ↑ 26,0 26,1 Ahmad V. U. Chemical Constituents of Some Medicinal Plants of Pakistan — 2011. — doi:10.1007/978-3-642-71425-2_1
- ↑ Luu K. T., Matches A. G., Nelson C. J. et al. Characterization of Inhibitory Substances of Tall Fescue on Birdsfoot Trefoil // Crop Science — 2010. — ISSN 0011-183X; 1435-0653 — doi:10.2135/CROPSCI1989.0011183X002900020034X
- ↑ DS M., DQ Y., SS Y. New Quinoid Glycosides from Forsythia suspensa // J. Nat. Prod. — ACS, 1998. — ISSN 0163-3864; 1520-6025 — doi:10.1021/NP970369A — PMID:9548879
- ↑ Li C., Dai Y., Zhang S. et al. Quinoid glycosides from Forsythia suspensa // Phytochemistry — Elsevier BV, 2014. — 9 p. — ISSN 0031-9422; 1873-3700 — doi:10.1016/J.PHYTOCHEM.2014.04.010 — PMID:24833035
- ↑ Mussinan C. J., Walradt J. P. Organic acids from fresh California strawberries // J. Agric. Food Chem. — USA: ACS, 1975. — ISSN 0021-8561; 1520-5118 — doi:10.1021/JF60199A018 — PMID:1150994
- ↑ Picha D. H. Organic acid determination in sweet potatoes by HPLC // J. Agric. Food Chem. — USA: ACS, 2005. — ISSN 0021-8561; 1520-5118 — doi:10.1021/JF00064A045
- ↑ Finnegan R. A., Patel J. K. Constituents of Mammea americana L. Part X. The isolation of some mono- and di-hydroxyxanthones. Observations on the synthesis of 1,5-,3,5-,1,6-, and 1,7-dihydroxyxanthone // Journal of the Chemical Society. Perkin transactions 1 — 1972. — ISSN 0300-922X; 2050-8255 — doi:10.1039/P19720001896
- ↑ Study on chemical constituents of Inula cappa // 中国中药杂志 — 2015. — ISSN 1001-5302 — doi:10.4268/CJCMM20150419
- ↑ 34,0 34,1 Mašterov I., Suchý V., Uhrín D. et al. Homoisoflavanones and other constituents from Muscari racemosum // Phytochemistry — Elsevier BV, 1991. — ISSN 0031-9422; 1873-3700 — doi:10.1016/0031-9422(91)83764-C
- ↑ Yang S., Shen T., Zhao L. et al. Chemical constituents of Lobelia chinensis // Fitoterapia — Elsevier BV, 2014. — 7 p. — ISSN 0367-326X; 1873-6971; 1971-551X — doi:10.1016/J.FITOTE.2014.01.007 — PMID:24444893
- ↑ NUMATA A., HOKIMOTO K., TAKEMURA T. et al. Plant constituents biologically active to insects. V. Antifeedants for the larvae of the yellow butterfly, Eurema hecabe mandarina, in Osmunda japonica. // Chemical & Pharmaceutical Bulletin — Pharmaceutical Society of Japan, 1984. — ISSN 0009-2363; 1347-5223 — doi:10.1248/CPB.32.2815
- ↑ Kang H. S., Choi J. H., Cho W. K. et al. A sphingolipid and tyrosinase inhibitors from the fruiting body of Phellinus linteus // Archives of Pharmacal Research — Springer Science+Business Media, Springer Nature, Pharmaceutical Society of Korea, 2004. — ISSN 0253-6269; 1976-3786 — doi:10.1007/BF02980143 — PMID:15357002
- ↑ Barrero A. F., Oltra J. E., Poyatos J. A. Acidic metabolites from Phycomyces blakesleeanus // Phytochemistry — Elsevier BV, 1996. — ISSN 0031-9422; 1873-3700 — doi:10.1016/0031-9422(96)00146-X
- ↑ KAZUNO C., MIURA H. Studies on constituent of edible fungi. Part II. Chemical constituents of Pleurotus ostreatus. // 日本食品工業学会誌 — 2011. — ISSN 0029-0394 — doi:10.3136/NSKKK1962.32.338
- ↑ W. Greenaway, T. Scaysbrook, F.R. Whatley Phenolic analysis of bud exudate of Populus lasiocarpa by GC/MS // Phytochemistry — Elsevier BV, 2002. — 3 p. — ISSN 0031-9422; 1873-3700 — doi:10.1016/0031-9422(88)80758-1
- ↑ W. Greenaway Compositions of Bud and Leaf Exudates of Some Populus Species Compared // Z. Naturforsch. C Bio. Sci. / J. Seibel — De Gruyter, 2018. — ISSN 0939-5075; 1865-7125 — doi:10.1515/ZNC-1992-0602
- ↑ Poyrazoğlu E., Gökmen V., Artιk N. Organic Acids and Phenolic Compounds in Pomegranates (Punica granatum L.) Grown in Turkey // J. Food Comp. Anal. — Elsevier BV, 2005. — ISSN 0889-1575; 1096-0481 — doi:10.1006/JFCA.2002.1071
- ↑ Lansky E. P., Newman R. A. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer // J. Ethnopharmacol. — Elsevier BV, 2007. — 30 p. — ISSN 0378-8741; 1872-7573 — doi:10.1016/J.JEP.2006.09.006 — PMID:17157465
- ↑ Habashi R., Hacham Y., Dhakarey R. et al. Elucidating the role of shikimate dehydrogenase in controlling the production of anthocyanins and hydrolysable tannins in the outer peels of pomegranate // BMC Plant Biology — BMC, Springer Science+Business Media, 2019. — ISSN 1471-2229 — doi:10.1186/S12870-019-2042-1 — PMID:31694546
- ↑ Wang X. Q., Peng Y., Peng B. et al. Chemical Constituents of Paraboea glutinosa // Chemistry of Natural Compounds — Springer Science+Business Media, 2014. — ISSN 0009-3130; 1573-8388 — doi:10.1007/S10600-014-1130-6
- ↑ Morota T., Nishimura H., Sasaki H. et al. Five cyclopentanoid monoterpenes from Rehmannia glutinosa // Phytochemistry — Elsevier BV, 1989. — ISSN 0031-9422; 1873-3700 — doi:10.1016/S0031-9422(00)97989-5
- ↑ Chimnoi N. Constituents of the leaves of Macaranga tanarius // J. Nat. Prod. — ACS, 2005. — ISSN 0163-3864; 1520-6025 — doi:10.1021/NP0500272 — PMID:15974621
- ↑ Shilling D. G., Jones L. A., A. Douglas Worsham et al. Isolation and identification of some phytotoxic compounds from aqueous extracts of rye (Secale cereale L.) // J. Agric. Food Chem. — USA: ACS, 2005. — ISSN 0021-8561; 1520-5118 — doi:10.1021/JF00070A011
- ↑ Wang L., Hu L. Chemical Constituents of Siegesbeckia orientalis L. // Journal of Integrative Plant Biology — Wiley-Blackwell, 2006. — ISSN 1672-9072; 1744-7909; 0577-7496 — doi:10.1111/J.1744-7909.2006.00279.X
- ↑ Liu M., Zhu G., Liang F. et al. [Studies on chemical constituents of rhizomes of Smilax trinervula]. // 中国中药杂志 — 2016. — ISSN 1001-5302 — doi:10.4268/CJCMM20160315 — PMID:28868862
- ↑ Wang R., Yang X., Ma C. et al. Alkaloids from the Seeds of Sterculia lychnophora (Pangdahai). // ChemInform — Wiley Information Services GmbH, Fachinformationszentrum Chemie GmbH (FIZ CHEMIE Berlin), 2005. — ISSN 0931-7597; 1522-2667; 1431-5890 — doi:10.1002/CHIN.200338184
- ↑ KM W., JL M., RL J. et al. Identification of 3-hydroxy-3-methylglutaric acid (HMG) as a hypoglycemic principle of Spanish moss (Tillandsia usneoides). // J. Nat. Prod. — ACS, 1995. — ISSN 0163-3864; 1520-6025 — doi:10.1021/NP50122A023 — PMID:7595594
- ↑ Nakatani M. A dihydroxycyclopentadienone and other constituents from the seeds of Trifolium repens // Phytochemistry — Elsevier BV, 1989. — ISSN 0031-9422; 1873-3700 — doi:10.1016/S0031-9422(00)98014-2
- ↑ T Hase Three iridoid glycosides from Viburnum furcatum // Phytochemistry — Elsevier BV, 1985. — ISSN 0031-9422; 1873-3700 — doi:10.1016/S0031-9422(00)81125-5
- ↑ Yang M., Kuo P., Hwang T. et al. Anti-inflammatory principles from Cordyceps sinensis // J. Nat. Prod. — ACS, 2011. — ISSN 0163-3864; 1520-6025 — doi:10.1021/NP100902F — PMID:21848266
- ↑ Jones O. Mixtures of similarly acting compounds in Daphnia magna: from gene to metabolite and beyond // Environ. Int. — Elsevier BV, 2010. — ISSN 0160-4120; 1873-6750 — doi:10.1016/J.ENVINT.2009.12.006 — PMID:20117838
- ↑ Wishart D. S., Mandal R. The human saliva metabolome // Metabolomics — Springer Science+Business Media, 2015. — ISSN 1573-3882; 1573-3890 — doi:10.1007/S11306-015-0840-5
- ↑ Gardiner N. J., Lakshmanan M., Martínez V. S. et al. Recon 2.2: from reconstruction to model of human metabolism // Metabolomics — Springer Science+Business Media, 2016. — ISSN 1573-3882; 1573-3890 — doi:10.1007/S11306-016-1051-4 — PMID:27358602
- ↑ Nielsen J., Westerhoff H., Kell D. et al. A community-driven global reconstruction of human metabolism // Nature Biotechnology — NPG, 2013. — ISSN 1087-0156; 1546-1696 — doi:10.1038/NBT.2488 — PMID:23455439
- ↑ SASAKI T. ÜBER DIE BESTANDTEILE DES ÄTHER-SOWIE ALKOHOLEXTRAKTS DES “MATSUDAKE”-PILZES (ARMILLARIA EDODES) // J. Biochem. — OUP, 1939. — ISSN 0021-924X; 1756-2651 — doi:10.1093/OXFORDJOURNALS.JBCHEM.A125810
- ↑ F.R. Stermitz, W.T. Lowry, F.A. Norris et al. Aliphatic nitro compounds from Astragalus species // Phytochemistry — Elsevier BV, 1972. — 8 p. — ISSN 0031-9422; 1873-3700 — doi:10.1016/S0031-9422(00)88463-0
- ↑ Ayer W. A., Browne L. M., Feng M. et al. The chemistry of the blue stain fungi. Part 1. Some metabolites of Ceratocystis species associated with mountain pine beetle infected lodgepole pine // Can. J. Chem. — NRC Research Press, 1986. — ISSN 0008-4042; 1480-3291 — doi:10.1139/V86-149
- ↑ Sakaki T., Ichihara A., Sakamura S. Isolation of Fulvic Acid fromCercospora beticola // Agricultural and biological chemistry — 1981. — ISSN 0002-1369; 1881-1280 — doi:10.1080/00021369.1981.10864692
- ↑ Zwenger C. Ueber Chelidoninsäure, eine neue Säure aus Chelidonium majus // Justus Liebigs Annalen der Chemie — 1860. — ISSN 0075-4617 — doi:10.1002/JLAC.18601140306
- ↑ Lee K. Y., Sung S. H., Kim Y. C. New Acetylcholinesterase-Inhibitory Pregnane Glycosides of Cynanchum atratum Roots // Helvetica Chimica Acta — Wiley, 2003. — ISSN 0018-019X; 1522-2675 — doi:10.1002/HLCA.200390047
- ↑ P KARRER, E MATTER Eine Untersuchung der sauren Bestandteile von Digitalis purpurea L // Helvetica Chimica Acta — Wiley, 1948. — ISSN 0018-019X; 1522-2675 — doi:10.1002/HLCA.19480310317 — PMID:18915716
- ↑ Thorsell W., Hasselquist H., Theander O. et al. Some Acids Belonging to the Citric Acid Cycle in the Liver Fluke, Fasciola hepatica, L. // Acta Chemica Scandinavica — RSC, 1963. — ISSN 0904-213X; 0001-5393 — doi:10.3891/ACTA.CHEM.SCAND.17-2129
- ↑ Schmalfuss H., Keitel K. Vorarbeiten für den Nachweis von Säuren in Pflanzen. 2. Mitteilung. Über Pflanzensäuren aus Glaucium und über dessen Blütenfarbstoffe. // Hoppe-Seyler's Zeitschrift für physiologische Chemie — B: Verlag Walter de Gruyter, 1924. — ISSN 0018-4888 — doi:10.1515/BCHM2.1924.138.3-6.156
- ↑ Dunstan W. R., Henry T. A. XVI.—The volatile constituents of the wood of Goupia tomentosa // Journal of the Chemical Society. Transactions — 1898. — ISSN 0368-1645; 2050-5450 — doi:10.1039/CT8987300226
- ↑ Olennikov D. N., Agafonova S. V., Nazarova A. V. et al. Organic acids and carbohydrates from Laetiporus sulphureus fruiting bodies // Chemistry of Natural Compounds — Springer Science+Business Media, 2008. — ISSN 0009-3130; 1573-8388 — doi:10.1007/S10600-009-9180-X
- ↑ DV R., EV R. Phytochemical investigations on the leaves of Notonia grandiflora // Planta Med. — Thieme Medical Publishers (Germany), 1972. — ISSN 0032-0943; 1439-0221 — doi:10.1055/S-0028-1099605 — PMID:5081820
- ↑ Wei W., Pan Y., Chen Y. et al. Carboxylic Acids from Phyllanthus urinaria // Chemistry of Natural Compounds — Springer Science+Business Media, 2005. — ISSN 0009-3130; 1573-8388 — doi:10.1007/S10600-005-0064-4
- ↑ Olennikov D. N., Mikhailova T. M., Tankhaeva L. M. et al. Organic Acids of Medicinal Plants. 1. Plantago major // Chemistry of Natural Compounds — Springer Science+Business Media, 2005. — ISSN 0009-3130; 1573-8388 — doi:10.1007/S10600-005-0180-1
- ↑ Franzen H., Helwert F. Über die chemischen Bestandteile grüner Pflanzen. 20. Mitteilung. Über die Säuren der Kirschen (Prunus avium). // Hoppe-Seyler's Zeitschrift für physiologische Chemie — B: Verlag Walter de Gruyter, 1922. — ISSN 0018-4888 — doi:10.1515/BCHM2.1922.122.1-3.46
